본문
Why Does Nature Choose Ca2+ Ion for Water Oxidation? A Hint from Reactivity Studies of Iron(III)-Peroxo Complexes Binding Redox-Inactive Metal Ions
Department of Chemistry and Nano Science
by Prof. Wonwoo Nam (wwnam@ewha.ac.kr)
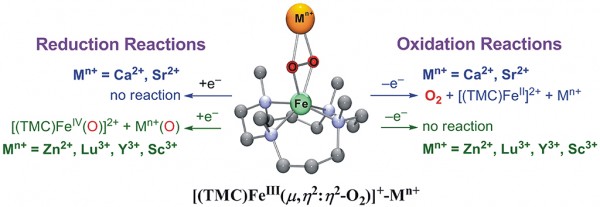
Photoinduced dioxygen activation by a nonheme iron complex, [(TMC)FeII]2+ (TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane), in the presence of redox-inactive metal ions (Mn+) and an electron donor [1-benzyl-1,4-dinitronicotinamide dimer, (BNA)2] resulted in the generation of two different intermediates, (TMC)FeIII-(μ,η2:η2-O2)-Mn+ (1-Mn+) and [(TMC)FeIV(O)]2+ (2), depending on the Lewis acidity of the redox-inactive metal ions. 1-Mn+ was formed when Mn+ was Ca2+ or Sr2+, whereas 2 was the product when Mn+ was Zn2+, Lu3+, Y3+ or Sc3+. Authentic 1-Mn+ complexes were synthesized independently and characterized with various spectroscopic methods, and their electrochemical properties and reactions with electron donor and acceptor were investigated. With respect to the electrochemical properties of 1-Mn+, we have observed a trend that as the Lewis acidity of Mn+ increases, both one-electron oxidation and reduction potentials of 1-Mn+ become more positive. When 1-Mn+ was reacted with reductant [e.g., (BNA)2], 1-Mn+ was converted to 2 in the case of Mn+ = Zn2+, Lu3+, Y3+ and Sc3+, but such a conversion was not observed in the case of Mn+ = Ca2+ and Sr2+. In the reaction with an oxidant such as cerium(IV) ammonium nitrate (CAN), 1-Mn+ reverted back to [FeII(TMC)]2+ by releasing O2 in the case of Mn+ = Ca2+ and Sr2+, but such a reaction did not occur in the case of Mn+ = Zn2+, Lu3+, Y3+ and Sc3+. The release of O2 in the reaction of 1-Ca2+ with CAN was confirmed using 18O-isotope labeled [(TMC)FeIII(18O2)]+-Ca2+ (or Sr2+) complexes. These results demonstrate that the Lewis acidity of the redox-inactive metal ions is an important factor that modulates not only the electrochemical properties of 1-Mn+ but also the reactivities of 1-Mn+ with electron donor and acceptor. We have discussed these results in the light of the functional role(s) of Ca2+ ion in the oxidation of water to dioxygen by the oxygen-evolving complex (OEC) in Photosystem II (PSII).
*Related article:
Redox-inactive metal ions modulate the reactivity and oxygen release of mononuclear non-haem iron(III)-peroxo complexes. Nature Chemistry. 6(10): 934–940 (2014)