본문
The role of TET-mediated hydroxymethylcytosine at the Pparγ locus in adipogenesis thickness
by Prof. Yoon Jung Park (park.yoonjung@ewha.ac.kr)
Department of Nutritional Science & Food Management
Adipose tissue plays a key role in regulation of energy homeostasis, not only through energy storage but also endocrine function such as adipokine secretion. Its dysfunction is closely associated with various metabolic disorders including obesity and type 2 diabetes. It is primarily composed of adipocytes, which differentiate from mesenchymal stem cells. The lineage commitment of MSCs to adipocytes is driven by the activation of master transcriptional regulators. PPARγ is a member of the nuclear receptor superfamily of ligand-activated transcription factors, cooperating with C/EBPα to act as a key driving force to induce adipogenesis. Understanding of the molecular mechanisms by which PPARγ regulates adipogenesis is critical to develop therapeutic strategies for the metabolic pathophysiology of obesity.
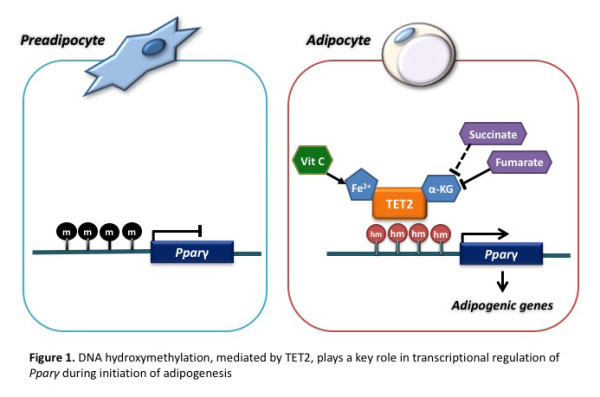
Recent evidence has shown that cell differentiation required proper epigenomic transition, beside serial coordination of adipogenic transcriptional factors. Epigenomic transition for cell differentiation and lineage decision takes place via changes of epigenetic patterns such as DNA methylation and histone modifications. DNA methylation is found in cytosines of the dinucleotide sequence CpG in mammals and has been demonstrated to be responsible for the stable maintenance of gene expression patterns during development or differentiation. Mammalian DNMTs are responsible for the acquisition of DNA methylation. The removal process of DNA methylation has not been well delineated. Recently, however, Tet methylcytosine dioxygenase (TET) protein family, composed of the three members TET1, TET2, and TET3 have the capacity to convert 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC) of DNA that is an intermediate for oxidative demethylation. They are Fe(II)- and a-ketoglutarate (α-KG)-dependent dioxygenases that successively convert 5-mC to 5-hmC, 5-formylcytosine, and 5-carboxycytosine of the DNA. Since adipogenesis is one of the most dynamic processes of cellular differentiation, the analysis of genome-wide or gene specific 5-hmC pattern during this process may clarify its underlying mechanisms. The role of histone marks during adipocyte differentiation has been actively explored, but DNA methylation or demethylation remains poorly investigated.
We investigated whether expression of the Tet genes and production of 5-hmC are required for preadipocyte differentiation using 3T3-L1 cell model. As results, both Tet1 and Tet2 genes were upregulated in a time-dependent manner upon adipogenic induction, accompanied by increased expression of hallmark adipogenic genes such as Pparγ and Fabp4. The TET upregulation led to reduced DNA methylation and elevated 5-hmC, both globally and specifically at the Pparγ locus. Knockdown of Tet1 and Tet2 blocked adipogenesis by repression of Pparγ expression. In particular, Tet2 knockdown repressed conversion of 5-mC to 5-hmC at the Pparγ locus. Moreover, vitamin C treatment enhanced adipogenesis, while fumarate treatment inhibited it by modulating TET activities. In conclusion, TET proteins, particularly TET2, were required for adipogenesis by modulating DNA methylation at the Pparγ locus, subsequently by inducing Pparγ gene expression.
* Related Article
Y Yoo, J H Park, C Weigel, D B Liesenfeld, D Weichenhan, C Plass, D-G Seo, A M Lindroth & Y J Park, TET-mediated hydroxymethylcytosine at the Pparγ locus is required for initiation of adipogenic differentiation, International Journal of Obesity, 2017 Apr; 41(4):652-659