본문
Interaction of tankyrase and a thiol peroxidase Prdx2 is indispensable for the survival of colorectal cancer cells
by Prof. Sang Won Kang (kangsw@ewha.ac.kr)
Highlights:
• Prdx2 is highly expressed in CRC.
• Prdx2 and tankyrase interacts only in the APC-mutant CRC cells.
• Loss of Prdx2 induces tankyrase inactivation and thus activates Axin1-mediated  -catenin destruction.
-catenin destruction.
• Inhibition of PrxII activity is sufficient to reduce the tumorigenic growth of APC-mutant CRC cells in vivo.
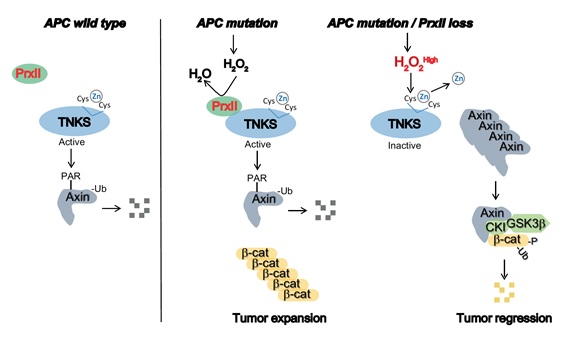
Colorectal cancer (CRC) is a cause of the highest cancer mortality in developed countries and is characterized by inactivating mutations of the adenomatous polyposis coli (APC) suppressor gene. APC is a key scaffold protein in the β-catenin destruction complex, which is composed of the axis inhibition protein 1 (Axin1), β-catenin, casein kinase 1 (CK1) and glycogen synthase kinase (GSK)-3β. Axis inhibition protein 1 (Axin1) tumor suppressor is another scaffold protein in the 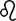 -catenin destruction complex, but endogenous Axin1 proteins are tightly controlled by tankyrase-dependent degradation in colorectal cancer cells. However, the molecular mechanism regulating tankyrase (TNKS) activity in CRC remains largely unknown. To date, numerous studies have shown that initiation of intestinal tumorigenesis by APC mutations is promoted by the acquired or inherited mutation in the DNA glycosylase enzymes essential for base excision repair of oxidative DNA damage, suggesting the involvement of elevated levels of reactive oxygen species (ROS) in driving the intestinal tumorigenesis driven by APC mutations. Mammalian 2-Cys Prx enzymes are actually the most efficient peroxidases regulating cellular ROS level. These proteins catalyze the reduction of H2O2 to water in the presence of NADPH by coupling with the thioredoxin/thioredoxin reductase system. It has been well established that 2-Cys Prx enzymes have multifaceted roles in cellular ROS detoxification and signal transduction. Therefore, we focused on elucidating a molecular mechanism involving TNKS and a thiol peroxidase named PrxII that may be necessary for the survival of CRC cells.
-catenin destruction complex, but endogenous Axin1 proteins are tightly controlled by tankyrase-dependent degradation in colorectal cancer cells. However, the molecular mechanism regulating tankyrase (TNKS) activity in CRC remains largely unknown. To date, numerous studies have shown that initiation of intestinal tumorigenesis by APC mutations is promoted by the acquired or inherited mutation in the DNA glycosylase enzymes essential for base excision repair of oxidative DNA damage, suggesting the involvement of elevated levels of reactive oxygen species (ROS) in driving the intestinal tumorigenesis driven by APC mutations. Mammalian 2-Cys Prx enzymes are actually the most efficient peroxidases regulating cellular ROS level. These proteins catalyze the reduction of H2O2 to water in the presence of NADPH by coupling with the thioredoxin/thioredoxin reductase system. It has been well established that 2-Cys Prx enzymes have multifaceted roles in cellular ROS detoxification and signal transduction. Therefore, we focused on elucidating a molecular mechanism involving TNKS and a thiol peroxidase named PrxII that may be necessary for the survival of CRC cells.
ROS has been recognized to serve a double-edged function, where a moderate and transient induction of cellular ROS levels is undoubtedly required for hyper-proliferation of cancer cells due to a second messenger role in growth factor signaling. In contrast, excessive production of ROS can be mutagenic and cytotoxic due to oxidative damage of macromolecules. In this study, we showed that loss of PrxII elevated the level of ROS and unexpectedly inhibited intestinal tumorigenesis induced by APC mutations, which further confirmed that H2O2 levels regulated by PrxII are critical for the survival of epithelial cancer cells in mouse intestinal adenomas. In CRC cells with an APC mutation, stringent knockdown of PrxII peroxidase drastically reduced the levels of total and active (unphosphorylated) β-catenin via the canonical destruction complex. When APC is mutated, functional Axin1 becomes important for regulation of β-catenin levels. The level of Axin1 is known to be regulated by poly (ADP-ribose) polymerization (PARsylation) and sequential ubiquitination of the protein. Depletion of PrxII blocked PARsylation/ubiquitination of Axin1 without affecting total ubiquitination and caused the augmentation of functional Axin1-associated destruction complexes to degrade β-catenin, hence reversing the oncogenic phenotype conferred by the APC mutation. Taken together, these results suggested that PrxII-mediated regulation of H2O2 promotes epithelial cancer progression by targeting TNKS. This pathway does not require classical Wnt/β-catenin signalling, as APC mutations induce Wnt-independent accumulation of transcriptionally active β-catenin.
TNKS, the sole PARsylating enzyme for regulating the level of Axin proteins, specifically interacts with the glycine residue at position 116 of PrxII through its ankyrin repeat cluster (ARC) 4/5 domains. Endogenous Axin1 proteins are tightly controlled by TNKS-dependent degradation via PARsylation and subsequent ubiquitination in CRC cells. It was shown for the first time that absence of PrxII reduced oncogenic β-catenin in the adenomatous polyps as well as the APC-mutant CRC cells due to the Axin1-dependent β-catenin degradation. It was also an intriguing result that H2O2-dependent inactivation of TNKS1 PARP activity happened as a result of the loss of zinc ions from the PARP domains. Taken together, the results reveal a novel redox mechanism by which a zinc-binding motif, essential for the PARP activity of TNKS, is vulnerable to oxidation and requires the PrxII-dependent antioxidant shielding effect. Presently, there have been no isoform-specific chemical compounds that enable the inhibition of peroxidase activity of human PrxII. Conoidin A, a cell-permeable compound that covalently binds to parasitic PrxII, was tested and shown to be sufficiently inhibited PrxI and PrxII activities using a slightly different mechanism. Interestingly, conoidin A inhibited colony-forming growth in APC-mutant CRC cells, but did not have the same effect on APC-competent CRC cells. Conoidin A treatment against tumor xenografts derived from APC-mutant CRC cells significantly retarded tumor growth. These results implied that a PrxII inhibitor may be used as a new therapeutic tool against with APC-mutant CRCs.
Overall, our study provided intrinsic mechanistic evidence for a tumor-promoting role specific to a thiol peroxidase PrxII. On the basis of our finding, we propose that targeting of PrxII may exert specific and broad therapeutic potentials for treating familial adenomatous polyposis (FAP) and APC-mutant CRCs.
* Related article
Dong Hoon Kang, Doo Jae Lee, Sunmi Lee, So-Young Lee, Yukyung Jun, Yerin Kim, Youngeun Kim, Ju-Seog Lee, Dae-Kee Lee, Sanghyuk Lee, Eek-Hoon Jho, Dae-Yeul Yu, and Sang Won Kang, Interaction of Tankyrase and Peroxiredoxin II is Indispensable for the Survival of Colorectal Cancer Cells, Nature Communications. 2017 Jun 28; 8(1):40.
Kang DH, Lee DJ, Lee KW, Park YS, Lee JY, Lee SH, Koh YJ, Koh GY, Choi C, Yu DY, Kim J, Kang SW, Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells, Molecular Cell. 2011 Nov 18; 44(4):545-558.
Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, Bae YS, Lee BI, Rhee SG, Kang SW, Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II, Nature. 2005 May 19;435(7040):347-53.