본문
Development of Topoisomerase II Specific Catalytic Inhibitor, an Effective Strategy for Chemotherapy
by Prof. Youngjoo Kwon (ykwon@ewha.ac.kr)
Department of Pharmaceutical Sciences, College of Pharmacy
Topoisomerase is a nuclear enzyme that relaxes supercoiled DNA. Its functional implication makes topoisomerase engage in various types of cellular processes such as DNA replication, transcription, recombination, chromosomal condensation and segregation. Since topoisomerase plays an essential role in cellular survival, it has been regarded as one of the most popular targets in cancer therapy.
Topoisomerase inhibitors are commonly divided into two groups; poison and catalytic inhibitor. Topoisomerase poison damages cancer cells by stabilizing the transiently formed DNA-enzyme cleavable complex. Because of its severe DNA toxicity and critical side effects, such as secondary malignancy, caused by the formation of undesired truncated DNA, it has limitation in use. For this reason, topoisomerase inhibitor which inhibits catalytic activity of topoisomerase without stabilizing DNA-enzyme cleavable complex and forming truncated DNA, so called catalytic inhibitor, is necessary in topoisomerase-targeting cancer therapy. One of the drawbacks of developed catalytic inhibitors is topoisomerase inhibitory activity which is far weaker than those of topoisomerase poisons. Our group has continuously searched for catalytic inhibitors which have potent topoisomerase inhibitory activities
and minimize genetic toxicity.
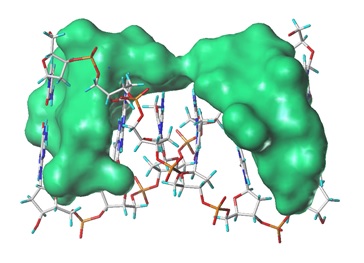
In our finding, a series of indeno-pyridine derivative and fluorescein hydrazones showed remarkable topoisomerase inhibitory activities. Systematic evaluation of topoisomerase inhibitory activity of TI-1-190, a indeno-pyridine derivative, was performed using various experimental methods and, consequently, clearly determined the mode of action. TI-1-190 selectively inhibited topoisomerase IIα by intercalating into double-stranded DNA. The activity of TI-1-190 was much stronger and the DNA toxicity was far less than those of etoposide, a clinically used topoisomerase II poison. Furthermore, TI-1-190 displays caspase 3-independent anticancer activity, which elucidates the effectiveness of TI-1-190 in caspase 3 deficient cancers.
Fluorescein hydrazones were newly synthesized with high yields. Using the topoisomerase relaxation assay and cancer cell viability assay, 3d and 3l were selected as a topoisomerase I and IIα dual inhibitor and a topoisomerase IIα specific inhibitor, respectively. Both compounds showed potent topoisomerase inhibitory activity without inducing DNA truncation. Based on the QSAR with the compounds, 45 fluorescein hydrazones were synthesized and evaluated for topoisomerase inhibitory activities. 5e, 5g, and 5h showed potent inhibitory activity against both topoisomerase I and IIα without DNA toxicity. In our observation, fluorescein hydrazones can be a novel scaffold for the development of antitumor agents targeting topoisomerase I and/or IIα catalytic activity with further optimizing process and the results will be explored in due courses.
* Related Article
Rahman AF, Park SE, Kadi AA, Kwon Y., Fluorescein hydrazones as novel nonintercalative topoisomerase catalytic inhibitors with low DNA toxicity, Journal of Medicinal Chemistry, 2014 Nov 13;57(21):9139-51.
Islam MS, Park S, Song C, Kadi AA, Kwon Y, Rahman AF, Fluorescein hydrazones: A series of novel non-intercalative topoisomerase IIα catalytic inhibitors induce G1 arrest and apoptosis in breast and colon cancer cells, European Journal of Medicinal Chemistry, 2017 Jan 5;125:49-67.
Jeon KH1, Park C, Kadayat TM, Shrestha A, Lee ES, Kwon Y., A novel indeno[1,2-b]pyridinone derivative, a DNA intercalative human topoisomerase IIα catalytic inhibitor, for caspase 3-independent anticancer activity, Chemical Communications, 2017 Jun 22;53(51):6864-6867.