본문
In-Situ Formation of Conductive Metal Sulfide Domain in Metal Oxide Matrix
by Prof. Seong-Ju Hwang (hwangsju@ewha.ac.kr)
Department of Chemistry and Nanoscience
Development of Economic Technology for Highly Efficient Electrode Material for Secondary Batteries
Lithium ion batteries (LIBs) receive prime attention as one of the most promising power sources for electric vehicle (EV) because of their long cycle life, high energy density, high work potential, and good rate capability. Spinel-structured Li4Ti5O12 attracts a great deal of research activity because of its highly stable operation potential of 1.55 V versus Li/Li+ and its excellent cyclability originating from negligible volume change during repeated Li+ insertionextraction process. The remarkable safety and reliability of the lithium titanate render this material one of the most suitable anode materials for the EV application. However, the rate performance of lithium titanate is seriously limited by its low electronic and ionic conductivities.
We develop a novel synthetic strategy of the in-situ formation of conductive metal sulfide domain to improve the low electronic and ionic conductivities of lithium titanate for the first time. This idea is based on the following two concepts. 1) In general, the metal sulfide possesses smaller bandgap energy and higher electrical conductivity than does the corresponding metal oxide, since (metal sulfur) bond is more covalent than (metal oxygen) bond. The composite formation with conductive metal sulfide can provide new efficient way to improve the electrode performance of semiconducting metal oxide through the increase of electrical conductivity. 2) Since the high toxicity of hydrogen sulfide might cause serious safety problems, less toxic CS2 liquid are used as a sulfurization agent to provide a safe and scalable synthetic method for metal oxidemetal sulfide nanocomposites.
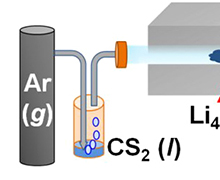
Finally, we are successful in developing a way to improve the electrode activity of lithium titanate on the basis of the in-situ formation of conductive metal sulfide (LixTiyS2) domain in the metal oxide (Li4Ti5O12) matrix using the CS2 liquid (Figure 1). All the present Li0.96Ti1.08S2-Li4Ti5O12 nanocomposites delivery large discharge capacities of ~143-160 mAhg-1 at 1 C rate, which are much greater than those of the pristine Li4Ti5O12 (~134 mAhg-1) and the reference LixTiyS2 (~115 mAhg-1). The present experimental findings clearly demonstrate that the in-situ formation of metal sulfide using CS2 is very effective in synthesizing novel nanocomposites consisting of intimately mixed metal sulfide and metal oxide domains and also in exploring promising composite electrode materials with good rate characteristics.
Of prime importance is that the universal applicability of the present method is confirmed from the improvement of the electrochemical activity of semiconducting CsTi2NbO7 upon the reaction with CS2. The current project of our group is the application of the present method for semiconducting metal oxidegraphene nanocomposites and nanostructured semiconducting metal oxides to develop high performance electrode materials for LIBs, supercapacitors, and other emerging electricity storage devices such as Na-ion and multivalent ion-batteries.
NOTE: This work has been selected as a cover paper in the issue of Advanced Functional Materials (Figure2).
Figure 1. Experimental scheme and photoimage of the highly efficient electrode materials of Li0.96Ti1.08S2Li4Ti5O12 for LIB.

Figure 2. Cover of the journal of Advanced Functional Materials (Vol. 25, issue 31).
* Related Article
In-Situ Formation of Conductive Metal Sulfide Domain in Metal Oxide Matrix: An Efficient Way to Improve the Electrochemical Activity of Semiconducting Metal Oxide, August 2015, Adv. Funct. Mater., Vol. 25, 4948-4955.