본문
Temperature-governed Performance of Practical CO2-containing Li–O2 Batteries
By prof. Dong Ha Kim,
Chemistry & Nanoscience
Research Profile
dhkim@ewha.ac.kr
Li–ion batteries, currently approaching their theoretical limits, are inadequate to answer upcoming energy requirements. Alternatively, the environment-friendly aprotic lithium–oxygen (Li–O2) battery offers high theoretical energy density (~3.5 kWh kg–1), based on the continuous formation/decomposition of lithium peroxide (Li2O2, E° = 2.96 VLi) during discharge/charge steps in the presence of high-purity O2. When directly exploiting practical air, however, CO2 traces and moisture were shown to fundamentally alter the O2 electrochemistry, inducing the formation of Li2CO3 and LiOOH/LiOH. The nucleophilic superoxide radical (O2–), formed via O2 reduction, preferentially reacts with the present CO2 in systems inducing strong Li+ solvation. The resulting peroxocarbonate species (CO4–, C2O62–) are reduced to solid-state Li2CO3 (4Li+ + O2 + 2CO2 + 4e– → 2Li2CO3), although their presence has also been uncovered at the end of discharge in tetraethylene glycol dimethyl ether (G4) and dimethyl sulfoxide-based solvents. The sustained peroxocarbonates are then oxidized during charge at <3.8 V with reversible O2/CO2 evolution, followed by the decomposition of Li2CO3 at higher overcharges (>4.2 V, absent O2 evolution).
In this work, we further focused on the practicality of CO2-containing Li–O2 batteries, which should be expected to perform under wide operating conditions. Representatively, commercial batteries for consumer usage undergo extensive temperature variations during operation, whereas military-grade and medically-oriented devices require stable performance even at –60 °C and 120 °C, respectively. We first revealed that operating temperatures (0~70 °C) critically dictate Li2CO3-associated cell overcharges (4.60~3.77 V), by determining temperature-dependent reaction kinetics and evolving species mobility, as analogously witnessed under pure O2-conditions (Figure 1). Against what is observed for the formation of Li2O2 in Li–O2 cells, however, cell temperatures did not govern the crystallinity of the Li2CO3 discharge product. In agreement with experimental observations, comprehensive density functional theory calculations also uncovered the effect of the temperature on the Li2CO3 precipitation mechanism and shed light on the dwindling stabilization of metastable peroxocarbonate intermediates during discharge at increasing cycling temperatures (Figure 2).
Focusing on the recharge step of these batteries, collected potentiostatic intermittent titration technique (PITT) responses revealed that at extremely low current cutoff conditions (3 μA), incremental potential steps rapidly reach a plateau at ~3.4 V, assigned to the oxidation of peroxocarbonates sustained in the electrolyte (Figure 3). These distinctly short steps (~12% of the charge depth) disclosed monotonic current decays and swiftly led to a rapid potential upshift to 3.75~3.85 V where the oxidation of Li2CO3 occurred. Our findings suggested that at these decreasing rates, a Li-deficient Li2C2O6 surface may undergo swift dissociation to carbonate species against kinetically-challenging oxidation steps yielding O2 and CO2. The disclosed slow and monotonic current decays during the entire charge plateau, a result in clear contrast with the decomposition of Li2O2, suggested that Li2CO3 oxidation is rate-limited by continuous electrochemical Li+ extraction steps: once the applied voltage overcomes the energy penalty to generate a Li-deficient surface, the resulting under-coordinated CO3 promotes a barrierless anion chemical extraction.
Another critical point of the practicality of these systems is their performance during extended operation. In particular, whereas the formation of Li2CO3 can be suppressed entirely at 0 °C, with ensuing cyclability, the peroxocarbonates could not be systematically sustained. At 0 °C the capacity was also dramatically decreased against that of the battery at room temperature, which showed that temperature-dependent reactants mobility in the highly-viscous G4 solvent should play a more prominent role under extended time periods. These results emphasize the need to further understand counteranion•••Li+•••solvent interaction degrees and the balance between mechanistic pathways in practical Li–air devices.
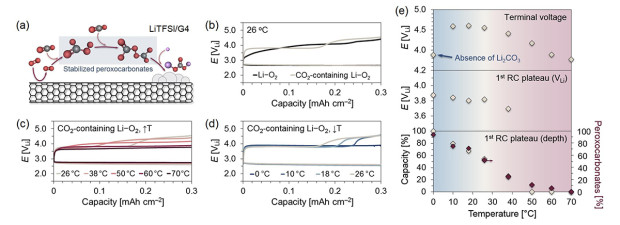
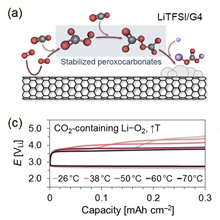
Figure 1. (a) Schematic representation of the discharge mechanism in the presence of CO2 in LiTFSI/G4. The superoxide radical reduced at the cathode surface reacts with CO2 and forms peroxocarbonate intermediates and solid-state Li2CO3. (b) Galvanostatic profiles of the first cycles of Li–O2 and CO2-containing Li–O2 (10% CO2) cells in 1.0 M LiTFSI/G4 at 26 °C. (c, d) Discharge/charge profiles of CO2-containing Li–O2 cells at increasing and decreasing temperatures in the 0~70 °C range. All galvanostatic tests were conducted at 0.06 mA cm–2 and a 0.3 mAh cm–2 capacity limit. (e) Comparison of the terminal voltage, and extent and potential of the 1st charge plateau (considered at <3.9 V) on the assessed temperature range. Comparison of corresponding mol % of stabilized peroxocarbonates in the preceding discharge step quantified through acid-base titrations.
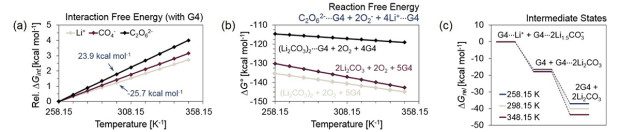
Figure 2. (a) The relative ΔGint is presented over a range of temperatures for the interactions between G4, and Li+ ion, CO4–, and C2O62–. ΔGint at 258.15 K is set to 0 kcal mol–1. (b) The reaction free energies are computed for three possible cases of G4 with: dimeric Li2CO3, monomeric Li2CO3 units, and non-interacting dimeric Li2CO3. (c) The free energies of intermediate states (relative to the initial state) of a possible lithiation pathway of peroxydicarbonate is depicted for three temperatures.
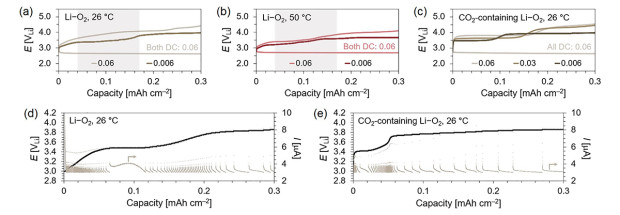
Figure 3. (a-c) Li–O2 and CO2-containing cells discharged at 0.06 mA cm–2 (fixed 0.3 mAh cm–2) and subsequently recharged at decreasing current rates at 26 and 50 oC. PITT response during recharge of (d) Li–O2 (20 mV steps) and (e) CO2-containing cells (10 mV) at 26 °C. Extremely low current cutoff-conditions (3 μA) were applied in each cases and charge was conducted up to 0.3 mAh cm–2.
* Related Article
Filipe Marques Mota, Omar Allam, Kyunghee Chae, Nur Aqlili Riana Che Mohamad, Seung Soon Jang, Dong Ha Kim, Practicality assessment: Temperature-governed performance of CO2-containing Li-O-2 batteries, Chemical Engineering, Journal Volume 449, December 2022