본문
Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via Notch1-WISP-1 signaling

By prof. Jihee Lee,
Department of Medicine
Research Profile
jihee@ewha.ac.kr
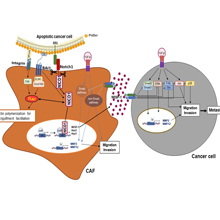
The interplay between apoptotic cancer cells and the tumor microenvironment modulates cancer progression and metastasis. Cancer-associated fibroblasts (CAFs) play a crucial role in promoting these events through paracrine communication. Here, we demonstrate that conditioned medium (CM) from lung CAFs exposed to apoptotic cancer cells suppresses TGF-β1-induced migration and invasion of cancer cells and CAFs. Direct exposure of CAFs to apoptotic 344SQ cells (ApoSQ) inhibited CAF migration and invasion and the expression of CAF activation markers. Enhanced secretion of Wnt‐induced signaling protein 1 (WISP-1) by CAFs exposed to ApoSQ was required for these anti-migration and -invasion effects. As expected, a lower concentration of rWISP-1 (≤10 ng/ml) had similar antimigratory and anti-invasive effects on 344SQ cells and CAFs. Furthermore, based on experiments using blocking antibodies against integrin α5, αv, β1, β3, or β5, our data suggest that integrin ανβ3 in 344SQ cells and ανβ5 in CAFs act as functional receptors for WIPS-1. Pharmacologic inhibition of Notch1 activation or siRNA-mediated Notch1 silencing prevented WISP-1 production by CAFs and reversed the antimigratory and anti-invasive effects. Enhanced expression of the Notch ligand delta-like protein 1 on the surface of ultraviolet-irradiated apoptotic lung cancer cells triggered Notch1-WSIP-1 signaling. Phosphatidylserine receptor brain-specific angiogenesis inhibitor 1 (BAI1)-Rac1 signaling, which facilitated efferocytosis by CAFs, participated in crosstalk with Notch1 signaling for optimal production of WISP-1.
In addition, a single injection of ApoSQ enhanced WISP-1 production, suppressed the expression of CAF activation markers in isolated Thy1+ CAFs, and inhibited lung metastasis in syngeneic immunocompetent mice via Notch1 signaling. Treatment with CM from CAFs exposed to ApoSQ suppressed tumor growth and lung metastasis, whereas treatment with WISP-1-immunodepleted CM from CAFs exposed to ApoSQ reversed the anti-tumorigenesis and -metastatic effects. Similarly, intratumoral injection of rWIPS-1 resulted in these antitumorigenic and antimetastatic effects. Thus, apoptotic cancer cell therapy targeting CAFs or cell-free therapies, such as treatment with CM from CAFs exposed to apoptotic cancer cells and CM components such as WIPS-1, could be effective therapeutic approaches to inhibit lung cancer progression and metastasis.
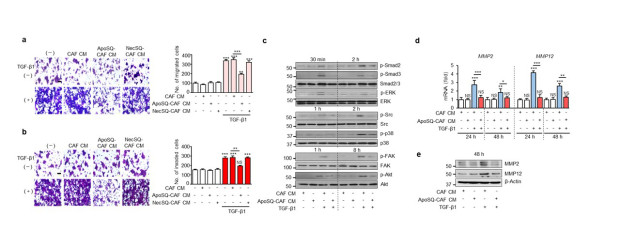
Figure 1. CM from CAFs exposed to apoptotic lung cancer cells inhibits TGF-β1 signaling pathways and the migration and invasion of lung cancer cells. (a) Phase-contrast microscopy (left) and quantification of migrated 344SQ cells (right) using Fn-coated Transwell plates. (b) Phase-contrast microscopy (left) and quantification of invaded 344SQ cells (right) (c) Immunoblot analysis of the indicated proteins in 344SQ cell lysates. (d) qRT-PCR of MMP2 and MMP12 in 344SQ samples. (e) Immunoblot analysis of MMP2 and MMP12 in 344SQ cell lysates.
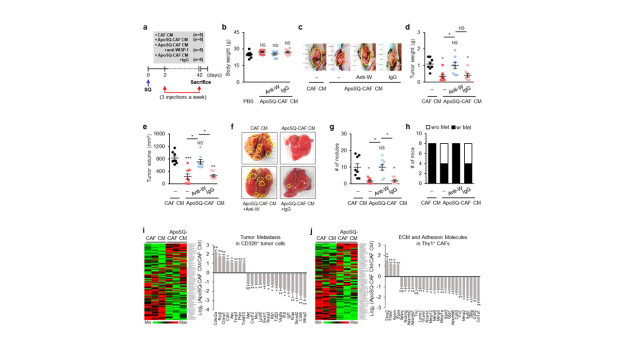
Figure 2. ApoSQ-CAF CM inhibits tumor growth and lung metastasis via WISP-1 in mice. (a) Schematic of experimental design and groups. CAF CM, ApoSQ-CAF CM, ApoSQ-CAF CM + anti-WISP-1, or ApoSQ-CAF CM + IgG was intratumorally injected three times a week for 6 weeks starting 2 days after subcutaneous injection of 344SQ cells into syngeneic (129/S) mice. Mice were necropsied 6 weeks later. Scatter plots of body weight (b), primary tumor weight (d), tumor volume (e), and numbers of lung metastatic nodules (g). (c) Representative images of primary tumors (yellow dashed circles). (f) Representative images of lungs with or without metastases. The yellow dashed circles indicate lung metastatic nodules. (h) Numbers of mice with (w/) or without (w/o) visibly determined metastases (Met). (i) Heatmap showing differentially expressed tumor metastasis-related genes in CD326+ tumor cells isolated from primary tumors (left). Relative expression of selected genes from PCR array profiling of tumor metastasis (right). Log2 fold-change values (ApoSQ-CAF CM vs. CAF CM). (j) Heatmap showing differentially expressed genes encoding ECM and adhesion molecules in Thy1+ CAFs isolated from primary tumors (left). Relative expression levels of selected genes from PCR array profiling (right).
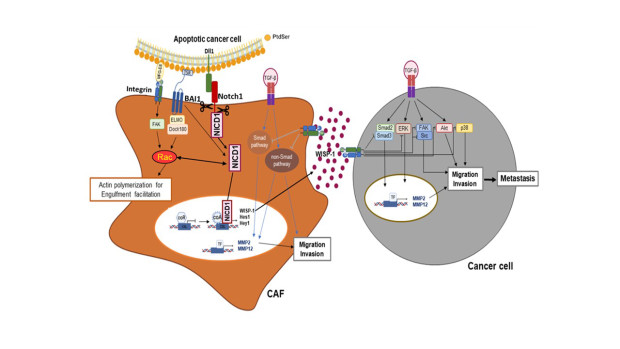
Figure 3. A schematic diagram summarizing and integrating the anti-metastatic effects by interaction of apoptotic cancer cells and cancer-associated fibroblasts in tumor microenvironment.
* Related Article
Hee Ja Kim, Kyungwon Yang, Kiyoon Kim, Ye‐Ji Lee, Sieun Lee, Sung Yong Ahn, Young‐Ho Ahn, Jihee Lee, Reprogramming of cancer-associated fibroblasts by apoptotic cancer cells inhibits lung metastasis via Notch1-WISP-1 signaling, CELLULAR & MOLECULAR IMMUNOLOGY, October 2022