본문
Targeting the HER2-ELF3-KRAS axis: a novel therapeutic strategy for KRASG13D colorectal cancer


By prof. So-Yeon Park & prof. Youngjoo Kwon
Department of Pharmaceutical Sciences
PURE Research Profile- Prof. So-Yeon Park & Prof. Youngjoo Kwon
& Prof. Youngjoo Kwon
syeonp@ewha.ac.kr & ykwon@ewha.ac.kr
This study identifies a previously unrecognized oncogenic regulatory network that drives aggressive tumor behavior and therapeutic resistance in a specific subtype of colorectal cancer. Published in Molecular Cancer, the work proposes a novel precision therapeutic strategy for KRASG13D-mutant colorectal cancer, a clinically challenging subgroup associated with poor prognosis.
Colorectal cancer is one of the most common malignancies worldwide, and mutations in the KRAS gene represent a major barrier to effective targeted therapy. Among KRAS variants, the G13D mutation exhibits distinct biological features and unpredictable responses to anti-EGFR therapies such as cetuximab. Despite its clinical relevance, the molecular mechanisms underlying this unique behavior have remained largely unclear.
In this study, we discovered a KRASG13D-specific transcriptional axis composed of HER2, the epithelial transcription factor ELF3, and KRAS itself. We demonstrate that HER2 overexpression activates ELF3, which directly enhances KRAS transcription, forming a self-reinforcing oncogenic circuit that promotes tumor growth, epithelial–mesenchymal transition (EMT), and resistance to cetuximab (Figure 1). Importantly, this regulatory axis operates selectively in KRASG13D-mutant tumors, but not in other KRAS subtypes. Notably, conventional HER2-targeted therapies, such as monoclonal antibodies, failed to suppress this pathway, highlighting a critical limitation of current treatment strategies. To overcome this challenge, we developed YK1, a novel small-molecule inhibitor that disrupts the ELF3–MED23 protein–protein interaction, thereby transcriptionally downregulating HER2 and KRAS. Treatment with YK1 effectively attenuated oncogenic signaling, reversed EMT-associated phenotypes, and resensitized KRASG13D colorectal cancer cells to cetuximab in both in vitro and in vivo models (Figure 2). Collectively, these findings provide compelling evidence that transcriptional suppression of HER2, rather than inhibition of the already overexpressed receptor at the protein level, represents a fundamentally more effective therapeutic strategy for KRASG13D colorectal cancer. This work not only deepen our understanding of KRAS mutation–specific tumor biology but also offer a promising new avenue for precision medicine.
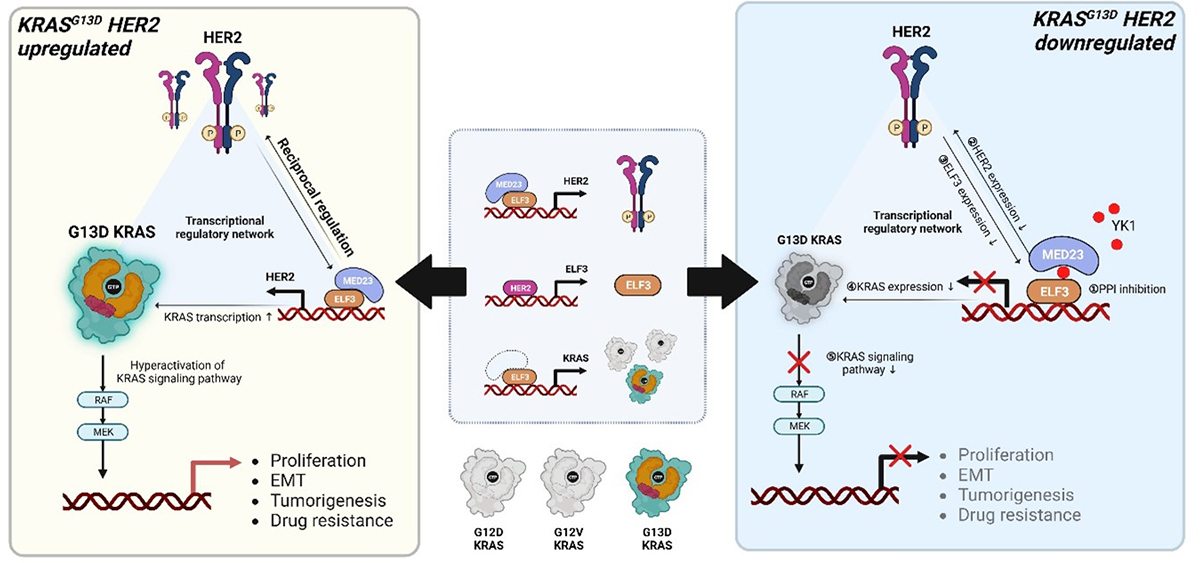
Figure 1. Graphical abstract
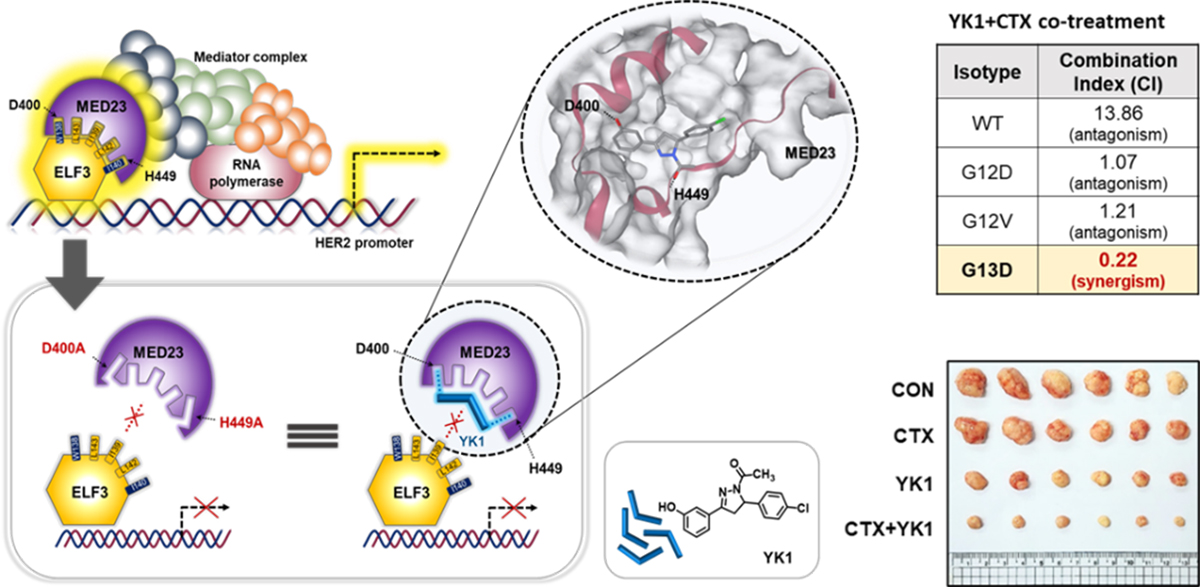
Figure 2. YK1 targeting ELF3-MED223 binding hotspot and synergistic effect of YK1 when combined cetuximab.
This research was supported by the STEAM research program, entitled “Development of an organoid phenocopy system–AI–based drug response prediction model and novel targeted therapeutics.”
* Related Article
1. Eun Seon Pak, Sehyun Jung, Hyeyoon Kim, Kyung‑Hwa Jeon, Seung Hee Seo, Inyoung Sung, Heetak Lee, So‑Yeon Park, Younghwa Na, Tae Il Kim, Youngjoo Kwon, Targeting the HER2‑ELF3‑KRAS axis: a novel therapeutic strategy for KRASG13D colorectal cancer, Molecular Cancer, 24, 139, 2025
2. Soo-Yeon Hwang, Seojeong Park, Hyunji Jo, Seung Hee Seo, Kyung-Hwa Jeon, Seojeong Kim, Ah-Reum Jung, Chanju Song, Misun Ahn, Soo Yeon Kwak, Hwa-Jong Lee, Motonari Uesugi, Younghwa Na, Youngjoo Kwon, Interrupting specific hydrogen bonds between ELF3 and MED23 as an alternative drug resistance-free strategy for HER2-overexpressing cancers, Journal of Advanced Research 47, 173-187, 2023