본문
Hyaluronic acid-bilirubin nanomedicine for targeted-modulation of dysregulated intestinal barrier, microbiome, and immune responses in colitis
By Prof. Yonghyun Lee (y.lee@ewha.ac.kr)
Department of Pharmacy
Pathogenesis of inflammatory bowel disease (IBD) is associated with disrupted intestinal barrier functions, dysbiosis of gut commensal microbiota, and subsequent dysregulated mucosal immune responses to gut microbiome. While traditional therapeutic approaches have mainly focused on treating the symptoms of IBD by suppressing dysregulated immune responses, they generally do not address the underlying causes of IBD, such as intestinal barrier functions and gut commensal bacteria. Moreover, they can cause off-target systemic side effects and serious complications. Towards the goal of addressing this unmet medical need, we have developed a new oral nanomedicine, hyaluronic-acid bilirubin nanoparticle (HABN) that can ameliorate colitis by targeted-modulation of gut microbiome, intestinal barrier functions, and the immune system.
In a murine model of colitis induced by 3% dextran sulfate sodium treatment, orally administered HABN accumulated in DSS-damaged colonic epithelium and pro-inflammatory macrophages and upregulated the expression levels of tight junction-associated proteins and anti-microbial peptides in colon, while restoring intestinal barrier functions and protecting the epithelial layer against apoptosis (Figure 1a). Additionally, HABN treatment had a potent immune-modulatory impact in the lamina propria, characterized by significant decreases in pro-inflammatory cytokines and immune cells with concomitant increases in anti-inflammatory factors (Figure 1a). Moreover, HABN promoted rapid recovery from bodyweight loss, inhibited colon and mucosa damage, and decreased colonic MPO activity in the DSS murine model of acute colitis (Figure 1b), whereas other control groups, including free HA (hyaluronic acid) mixed with bilirubin, HACN (Hyaluronic acid-cholesterol nanoparticles), as well as an oxidized form of HABN, HAoxBR, failed to protect animals against colitis. These results show the efficacy and safety of HABN and demonstrate that both the HA shell and the BN core play critical and complementary roles in multi-faceted benefits of HABN against colitis.
HABN also altered the gut microbiome, increasing the diversity and relative abundance of Akkermansia muciniphila, Clostridium XIV and Lactobacillus (Figure 1a, c). In particular, IBD patients have dramatically decreased abundance of Akkermansia muciniphila, known to promote mucus production and expression of tight junction proteins; Clostridium XIV whose metabolic byproduct, butyrate, induces Treg cells in lamina propria; and Lactobacillus, known to exert anti-inflammatory effects in both animal models of colitis and IBD patients. Notably, when we depleted commensal gut microbes with broad-spectrum oral antibiotics, HABN therapy partially lost its efficacy against DSS-colitis (Figure 1b). Overall, these results suggested that ROS-responsive and hyaluronidase-resistant HABN accumulated in DSS-inflamed colon and protected colonic epithelium against inflammation while restoring disrupted intestinal barrier functions and gut microbiome (Figure 1a).
Our work represents, to the best of our knowledge, the first demonstration of oral nanomedicine capable of targeting inflamed colon and directly modulating the intestinal barrier and gut microbiota while exerting potent anti-inflammatory responses against colitis. As dysregulated gut microbiota and intestinal barrier functions are highly associated with systemic diseases, our strategy described here may offer a convenient yet powerful platform for treatment of other inflammatory diseases.
Keywords : Hyaluronic acid, bilirubin, nanomedicine, colitis, gut microbiome, intestinal barrier.
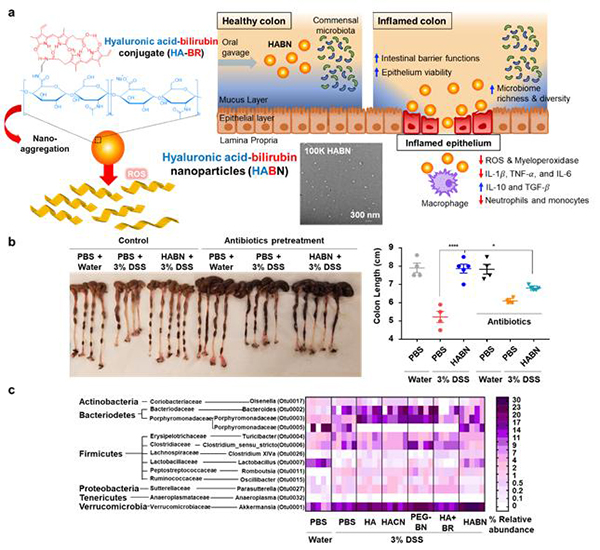
Figure 1. RHyaluronic acid-bilirubin nanomedicine (HABN) shows excellent anti-inflammation activity owing to targeted modulation of intestinal barrier, gut microbiome, and immune responses in colitis. (a) Schematic of HABN self-assembled from hyaluronic acid-bilirubin conjugate (HA-BR) and their TEM images. (b-c) Therapeutic efficacy of HABN against colitis shown in the (b) colonic length and (c) gut microbiome analysis.
* Related Article
Yonghyun Lee, Kohei Sugihara, Merritt G. Gilliland III, Sangyong Jon, Nobuhiko Kamada, and James J. Moon., Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome, and immune responses in colitis Nature Materials. 2020 Jan 1: 19 (1): 118-126.
- Featured by Nature Materials & New England Journal of Medicine