본문
N-terminus-independent activation of c-Src via binding to a tetraspan(in) TM4SF5 in hepatocellular carcinoma is abolished by the TM4SF5 C-terminal peptide application

By Prof. Sun Choi
College of Pharmacy & Graduate School of Pharmaceutical Sciences
PURE Research Profile
sunchoi@ewha.ac.kr
Active c-Src non-receptor tyrosine kinase localizes to the plasma membrane via N-terminal lipid modification. Membranous c-Src causes cancer initiation and progression. Even though transmembrane 4 L6 family member 5 (TM4SF5), a tetraspan(in), can be involved in this mechanism, the molecular and structural influence of TM4SF5 on c-Src remains elusive. Here, we investigated molecular and structural details by which TM4SF5 regulated c-Src devoid of its N-terminus and how cell-penetrating peptides could interrupt c-Src activation via interference of c-Src-TM4SF5 interaction in hepatocellular carcinoma models.
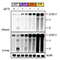
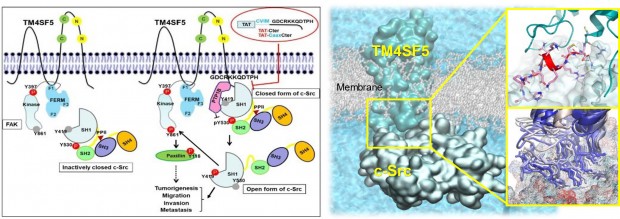
The C-terminal tail of TM4SF5 efficiently binds to the SH1 kinase domain of c-Src, especially to its inactively-closed form. The complex involved protein tyrosine phosphatase 1B able to dephosphorylate Tyr530. The c-Src SH1 domain alone, even in a closed form, bound TM4SF5 to cause c-Src Tyr419 and FAK Tyr861 phosphorylation. Especially, homology modeling and molecular dynamics simulation studies identified the atomic-level details of the binding process between TM4SF5 and c-Src. The residues predicted as a direct interface between the two proteins were further validated by point-mutation studies. Because this binding can be abolished by cell-penetrating peptides containing the TM4SF5 C-terminus, targeting this direct interaction may be an effective strategy for developing therapeutics that block the development and progression of hepatocellular carcinoma. The designed peptide blocked the interaction of TM4SF5 with c-Src and prevented c-Src-dependent tumor initiation and progression in vivo.
In summary, we newly found that the tumor protein c-Src can be activated at the cell membrane through its association with the membrane protein TM4SF5, even if it does not possess the ability to remain at the membrane, for example, by binding to fat molecules. We suggested a mechanism underlying hepatic fibrosis and hepatoma based on the signaling activation of these two proteins. This study will be expected to serve as a starting point in developing safe peptide biomedicines that can prevent the development of liver diseases, including hepatoma.
* Related Article
Haeng Eun Song, Yoonji Lee, Eunmi Kim, Chang Yun Cho, Oisun Jung, Doohyung Lee, Eun Goo Lee, Seo Hee Nam, Minkyung Kang, Stephani Joy Y Macalino, Ji Eon Kim, Jae Woo Jung, Sung Won Kwon, Sun Choi, Jung Weon Lee. N-terminus-independent activation of c-Src via binding to a tetraspan(in) TM4SF5 in hepatocellular carcinoma is abolished by the TM4SF5 C-terminal peptide application. Theranostics 2021, 11(16), 8092-8111.