본문
Recent advances in potassium metal batteries: electrodes, interfaces and electrolytes

By prof. Dong Ha Kim
Department of Chemistry & Nanoscience
PURE Research Profile
dhkim@ewha.ac.kr
Background and Motivation
Potassium metal batteries (PMBs) have emerged as promising next-generation energy storage systems owing to the high theoretical capacity of potassium metal anodes (687 mAh g⁻¹), low electrochemical potential, fast ion transport kinetics, and the natural abundance and low cost of potassium resources. Compared to lithium and sodium ions, K⁺ ions exhibit a smaller solvation radius in common organic electrolytes, enabling faster desolvation and migration, which is advantageous for high-power applications. Unlike potassium-ion batteries based on intercalation chemistry, PMBs rely on reversible potassium plating and stripping at the metal anode, enabling compatibility with high-capacity cathode systems such as sulfur and oxygen. However, despite these advantages, the practical implementation of PMBs is severely limited by the intrinsic instability of potassium metal anodes.
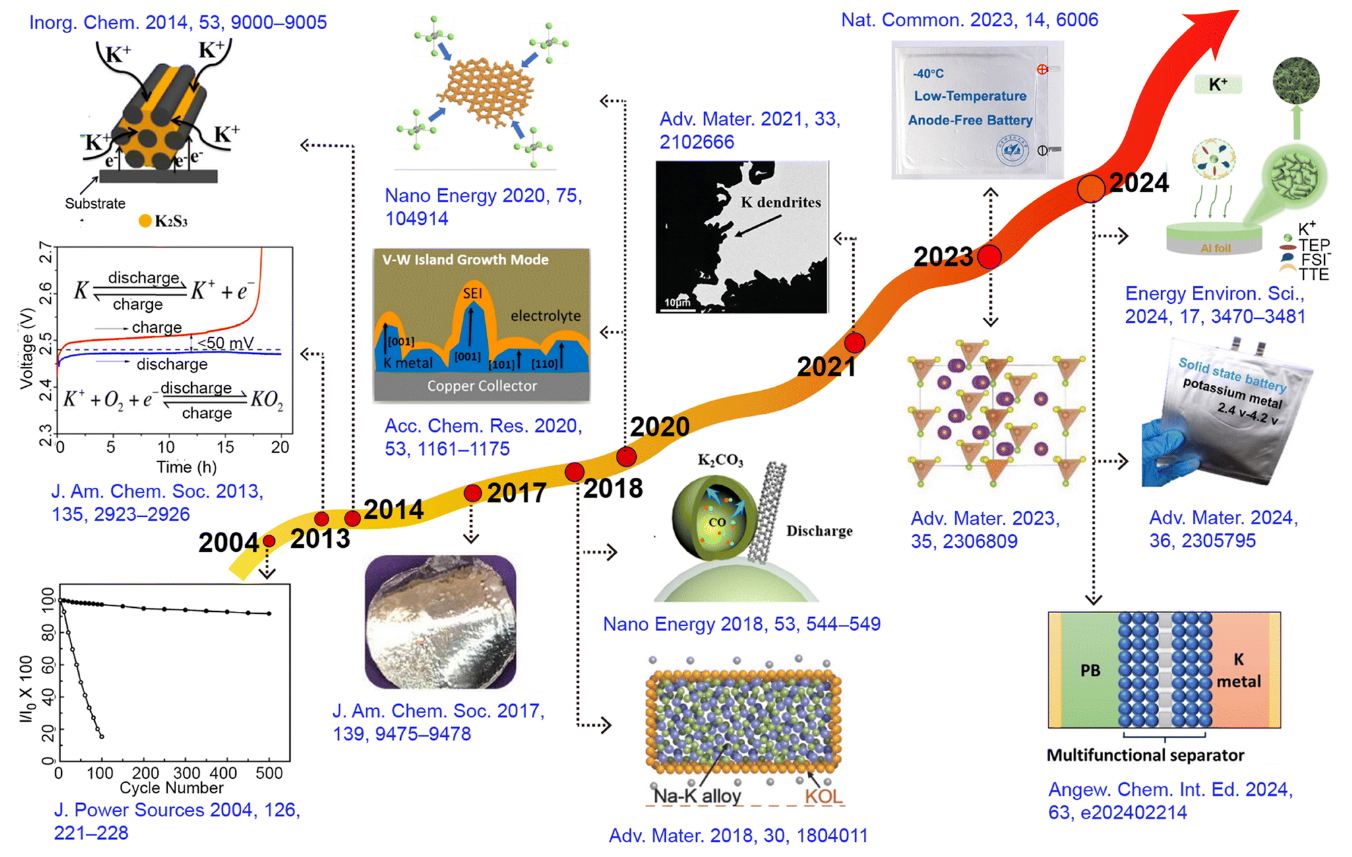
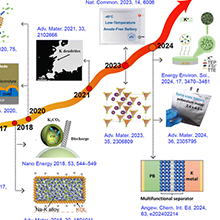
Figure 1. Timeline showing major developments in the field of PMBs: new electrode materials, separators, electrolytes, and theoretical concepts
Key Challenges in Potassium Metal Batteries
The major performance limitations of PMBs originate from the electrochemical behavior of potassium metal during cycling. Potassium ions are directly reduced on the anode surface, leading to non-uniform nucleation and irregular metal deposition. The high chemical reactivity and large volume change of potassium metal promote rapid dendrite growth, which continuously disrupts the solid electrolyte interphase (SEI). In addition, potassium-based SEI layers generally exhibit inferior mechanical stability and higher interfacial impedance compared to lithium-based counterparts, resulting in low Coulombic efficiency and rapid capacity fading.
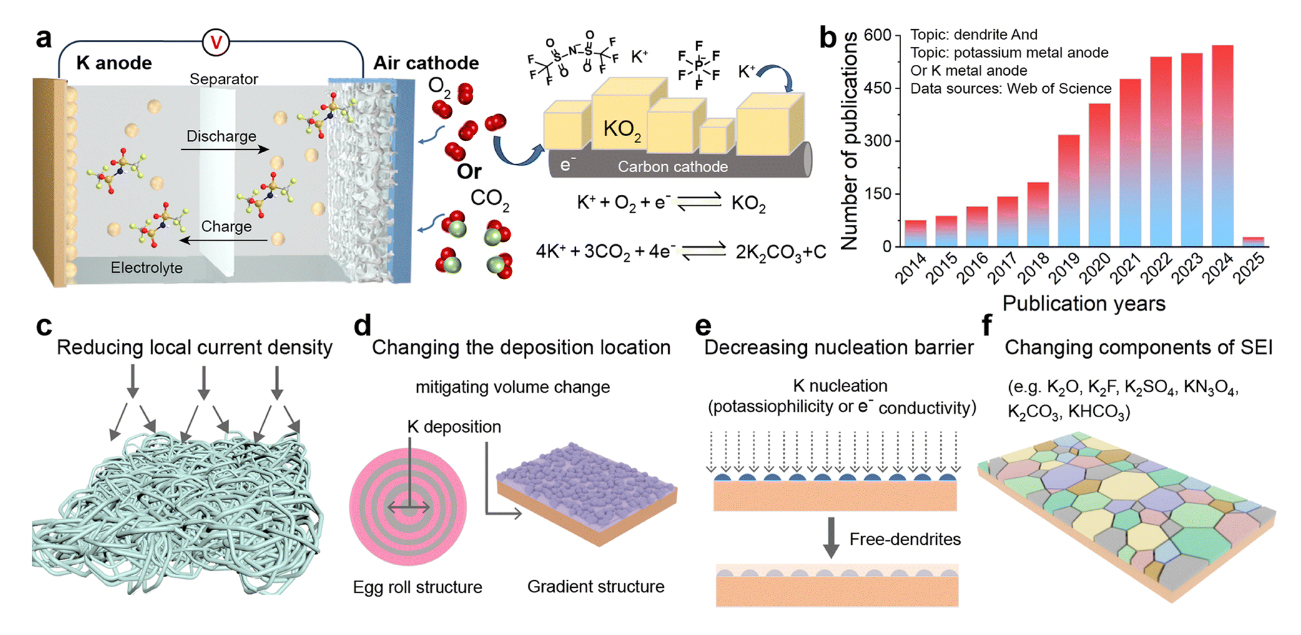
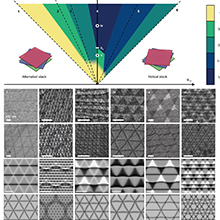
Figure 2. (a) Schematic illustration of potassium-air batteries. (b) Number of publications related to potassium metal anodes from 2014 to 2025. (c)–(f) Solutions for potassium metal anodes to address infinite volume changes and dendrite growth.
Strategies for Stabilizing Potassium Metal Anodes
To overcome the intrinsic instability of potassium metal anodes, recent studies have emphasized three interconnected strategies: electrode engineering, interface engineering, and electrolyte engineering, highlighting that effective stabilization requires their integrated design rather than isolated optimization. Electrode engineering focuses on regulating potassium nucleation and deposition by reducing local current density and homogenizing K⁺ flux, using approaches such as three-dimensional porous hosts, potassiophilic composite anodes, and liquid Na–K alloys, which can suppress dendrite growth but still face practical challenges in processing and sealing.
Interface and electrolyte engineering further stabilize the anode–electrolyte system by controlling interfacial chemistry and ion transport. Artificial SEI layers, functional interlayers, and modified separators enhance mechanical robustness and promote uniform potassium ion transport, while ether-based electrolytes, KFSI salts, localized high-concentration formulations, and solid-state electrolytes improve SEI stability, Coulombic efficiency, and safety, albeit with remaining limitations in ionic conductivity and interfacial compatibility.
Representative Potassium Metal Battery Systems
Beyond conventional full-cell configurations, PMBs have been extended to several high-energy battery systems. Potassium–sulfur batteries offer high theoretical energy density but suffer from polysulfide shuttle effects, complex reaction pathways, and electrolyte instability. Potassium–oxygen batteries are particularly notable due to their single-electron redox chemistry, forming KO₂ rather than Li₂O₂, which enables higher reversibility compared to lithium–oxygen systems. However, the chemical instability between superoxide species and electrolytes remains a critical challenge that must be addressed.
* Related Article
Jianlu Sun, Yichen Du, Yijiang Liu, Dongbo Yan, Xiaodong Li, Dong Ha Kim, Zhiqun Lin, Xiaosi Zhou, Recent advances in potassium metal batteries: electrodes, interfaces and electrolytes, Chemical Society Reviews, 2025