본문
Electrochemical CO2 removal from Oceanwater

By Prof. Seoni Kim
Department of Environmental Science and Engineering
seonikim@ewha.ac.kr
Research on efficiently removing carbon dioxide (CO₂) from the atmosphere has gained significant global attention to mitigate climate change. Recently, a promising approach to reducing atmospheric CO₂ levels involves directly extracting it from seawater. The ocean, being the primary absorber of CO₂ from the atmosphere, has historically absorbed around 30-40% of all anthropogenic CO₂ emissions
Conventional methods for CO₂ removal from seawater have utilized a bipolar ion exchange membrane stack, applying voltage and acidifying seawater through water electrolysis. In this process, bicarbonates in the water are converted into CO₂ molecules, which are then removed under vacuum conditions. However, challenges include the high cost of bipolar ion exchange membranes and increased complexity and costs associated with applying high voltages at both ends of the stack or introducing additional chemicals
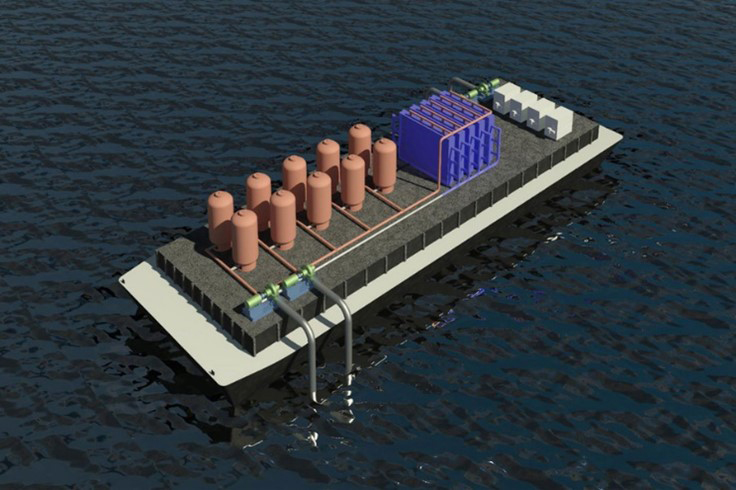
To address these issues, a collaborative research team led by Prof. Seoni Kim from the department of environmental science and engineering at Ewha Womans University, Prof. T. Alan Hatton from the department of Chemical Engineering at MIT, and Prof. Kripa B. Varanasi from the department of Mechanical Engineering at MIT has developed a novel system that does not require adding chemicals to the electrolyte and eliminates the need for a bipolar ion exchange membrane.
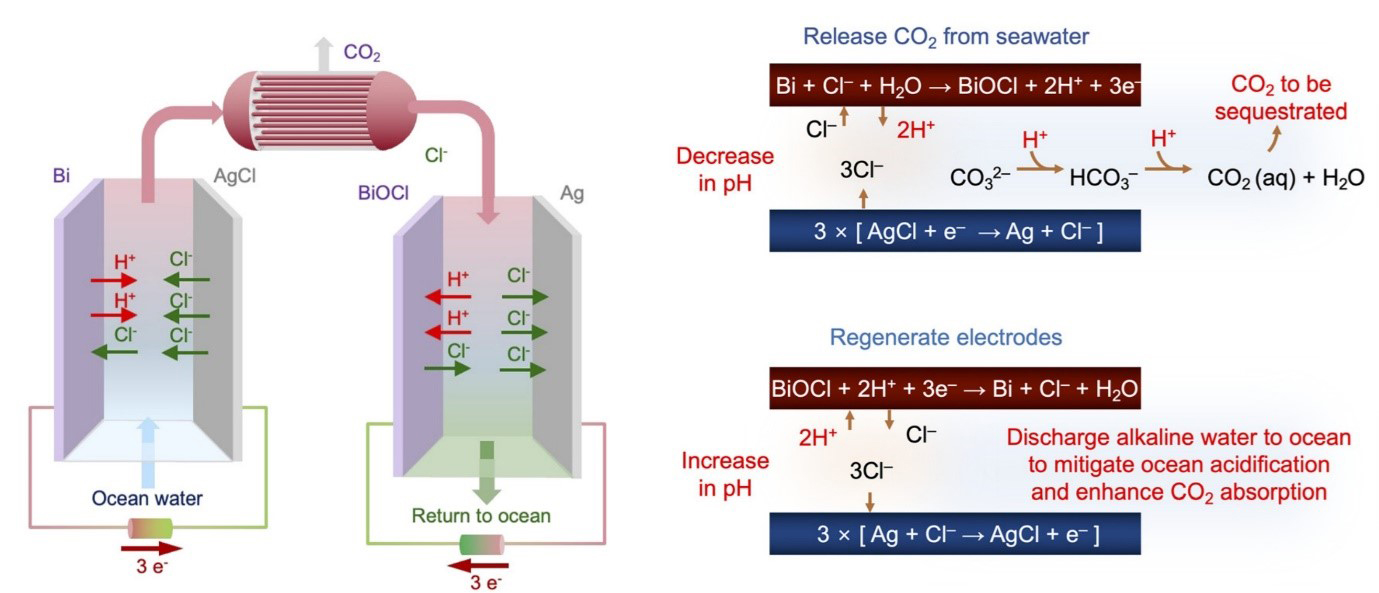
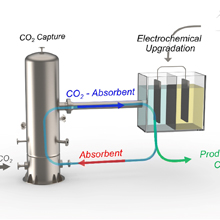
Figure General principle of the chloride-mediated electrochemical pH swing system for CO2 removal from ocean water on the left. Electrochemical reactions at bismuth (red) electrode and silver (blue) electrode in each step, and subsequent CO2 release in the acidified oceanwater on the right.
The devised process proved to be simpler in design compared to previously reported methods, yet demonstrated higher energy efficiency in capturing CO₂. In this process, the researchers first acidify seawater in the first cell, converting bicarbonates into CO₂, which is then collected in gas form. In the subsequent step, the water supplied to the second cell undergoes a reverse reaction to recover protons. The resulting acidic solution is then converted back to an alkaline state before being released back into the ocean. By repeating these steps, the system allows for continuous extraction of CO₂ from seawater.
* Related Article
Seoni Kim, Michael P. Nitzsche, Simon B. Rufer, Jack R. Lake, Kripa K. Varanasi, T. Alan Hatton, Asymmetric chloride-mediated electrochemical process for CO2 removal from oceanwater, Energy & Environmental Science, Energy & Environmental Science, Volume 16, Issue 5, pages 2023-2044, February 2023