본문
Integrated CO2 capture and electrochemical upgradation: the underpinning mechanism and techno-chemical analysis
by Prof. Dong Ha Kim
Chemistry & Nanoscience
PURE Research Profile
dhkim@ewha.ac.kr
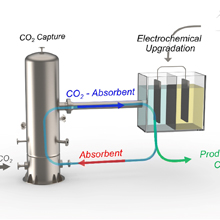
Marrying post-combustion CO2 capture with electrochemical utilization (CCU) represents a significant advancement in renewable energy science by eradicating the expenses and energy associated with transporting and storing CO2. Yet, the primary hurdles in implementing industrial-scale processes entail choosing a suitable solvent/electrolyte for CO2 capture, designing an infrastructure that links an electrolyzer to a CO2 point source along with a separator to isolate CO2 reduction reaction (CO2RR) products, and ultimately, determining the most fitting electrocatalyst. In this review, we highlight the major difficulties with detailed mechanistic interpretation in each step, to find out the underpinning mechanism involved in the integration of electrochemical CCU to achieve higher-value products (Figure 1). In the past decades, most of the studies dealt with individual parts of the integration process, i.e., either selecting a solvent for CO2 capture, designing an electrocatalyst, or choosing an ideal electrolyte. In this context, it is important to note that solvents such as monoethanolamine, bicarbonate, and ionic liquids are often used as electrolytes in CO2 capture media (Figure 2). Therefore, it is essential to fabricate a cost-effective electrolyzer that should function as a reversible binder with CO2 and an electron pool capable of recovering the solvent to electrolyte reversibly. For example, reversible ionic liquids, which are non-ionic in their normal forms, but produce ionic forms after CO2 capture, can be further reverted back to their original non-ionic forms after CO2 release with almost 100% efficiency through the chemical or thermal modulations. This review also sheds light on a focused techno-economic evolution for converting the electrochemically integrated CCU process from a pilot-scale project to industrial-scale implementation. In brief, this review article will summarize a state-of-the-art argumentation of challenges and outcomes over the different segments involved in electrochemically integrated CCU to stimulate urgent progress in the field.
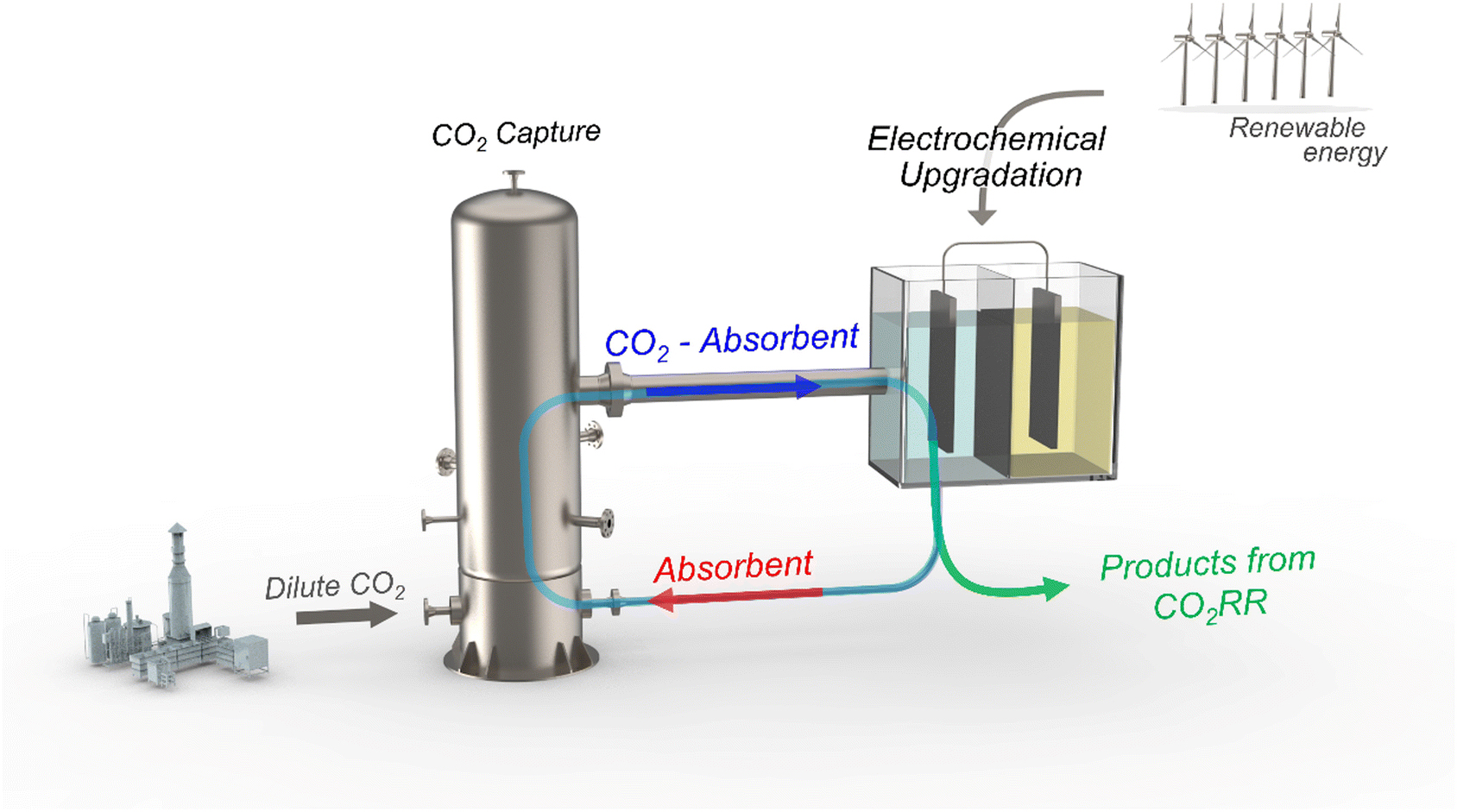
Figure 1. Schematic representation of integrated CO2 capture and its electrochemical upgradation from the dilute CO2 source. The CO2 absorbent is based on amine, bicarbonate, ILs and MOF/COF solvents.
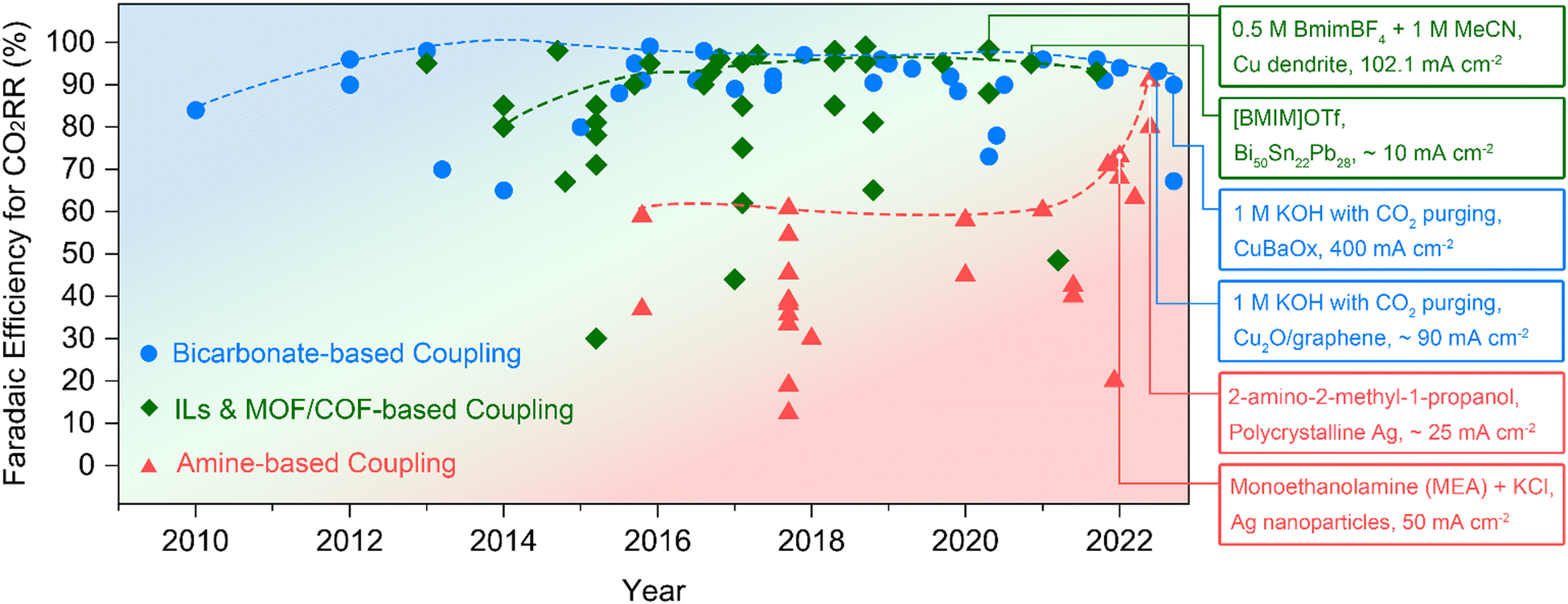
Figure 2. Progress in faradaic efficiencies for direct upgradation with CO2RR process which includes amine absorbent based, bicarbonate absorbent based, and ionic liquids and MOF/COF absorbent based electrochemical upgradation. BmimBF4: 1-butyl-3-methylimidazolium tetrafluoroborate. MeCN: acetonitrile. BMIM: 1-methyl-3-butyl-imidazolium.
* Related Article
Sandip Kumar De, Dong-Il Won, Jeongwon Kim, Dong Ha Kim, Integrated CO2 capture and electrochemical upgradation: the underpinning mechanism and techno-chemical analysis, Chemical Society Reviews, Vol. 52, Issue 16, 5744-5802, August 2023